|
Our objective is to provide services that correspond to your needs. All projects are performed according to the ICH-GCP standards, international regulatory requirements and ethics, and we guarantee complete confidentiality.
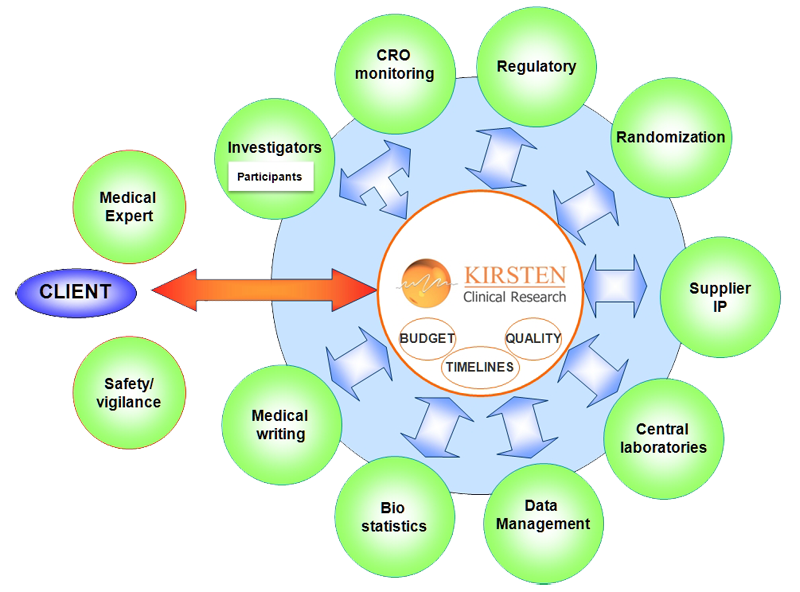
KIRSTEN CLINICAL RESEARCH can offer isolated tasks as well as an entire study coordination service.
Clinical study coordination
Specialized in the coordination of all third parties of the trial, we are committed to our 3 missions; providing quality services within the predefined timeframe and budget.
- A senior clinical project manager will be in charge of your project and your key contact person
- Scientific study design and preparation of essential and operational study documents
- Contract management with sponsors and subcontractors, including budget negotiation
- Management of clinical trial budget
- Ethics Committees & Competent Authorities submissions and notifications
- Coordination & motivation of recruitment in investigational sites, newsletters, organization of investigator meetings
- Quality assurance through set up of quality plans, performing co-monitoring visits, site & CRO audits
- Global study coordination and support of field based service providers (monitoring CROs, CRAs, randomization, data management, biometrics, drug suppliers, pharmaco-vigilance, central laboratories), with a continuous problem solving approach
- Tracking and reporting of project status and milestones to the sponsor
|
|
|
Monitoring
KIRSTEN CLINICAL RESEARCH offers high quality monitoring within the defined timelines for phase I to IV trials, including qualification, initiation, monitoring, and close-out visits to:
- Be the key contact person for the investigational sites and sponsor
- Stimulate the recruitment of subjects
- Verify source documentation and case report forms
- Manage data collection and resolve data queries within defined timelines
- Ensure the compliance to the study protocol and the good clinical practice (GCP) standards.
You want to know more, please click here
|
|